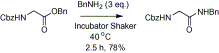Catalyst and solvent-free amidation of inactive esters of N-protected amino acids
Abstract
A catalyst free procedure for the preparation of amides from inactive esters of N-protected amino acids and various amines is demonstrated under mild reaction conditions. Our effort to recover excess amine and generated alcohol is an approach towards environment friendly and cost effective synthesis under easy operational conditions.
Keywords
- Aminolysis;
- Inactive esters;
- Amino acid;
- Amidation
Amides are one of the versatile functional groups in many organic and biomolecules.1 Amides derived from the reaction of amino acids and amines are important as they constitute a significant part of the chemical space of biologically active compounds. For example, N-(benzyloxycarbonyl)-glycine esters and amides exhibit anticonvulsant property. 2 Kohn and co-workers reported synthesis and anticonvulsant properties of some functionalized dl-amino acid derivatives as well as some N-benzylacetamido derivatives. 3
There exist many protocols in literature which describe their preparation either directly from acid or by derivatization of the acid into more reactive ester, anhydride or acid halide to form amide. Usually phenolic esters are considered as active esters as they react readily with amines at room temperature. On the other hand, alkyl esters are considered as inactive and widely used as protecting groups for carboxylic acid function especially in peptide chemistry. However, amidation from inactive alkyl esters is also reported under arduous conditions using various reagents. To name a few, ammonium chloride,4 sodium methoxide,5 sodium amide,6 sodium hydride,7 sodium cyanide,8 Grignard reagents,9 butyllithium,10 2-hydroxy pyridine,11 sodium 2- or 4-pyridinolate.12 Han et al. introduced use of group IV metal alkoxides in combination with additives like HOBt, HOAt etc. for amidation.13 In the same year Movassaghi and Schmidt reported the amidation of inactivated esters with amino alcohols using N-heterocyclic carbene as the catalyst. 14 Mioskowski achieved aminolysis of methyl esters using TBD (1,5,7-triazabicyclo [4.4.0] dec-5-ene) as catalyst. 15 Recently, catalytic activity of DBU (1,8-diazabicyclo [5.4.0] undec-7-ene) 16 and an equimolar mixture of DBU and 1,2,4-triazole 17 for such ester to amide exchange reactions has been demonstrated. Ammonolysis of inactive esters are also reported using Mg(OCH3)2/NH3, CaCl2/NH3 and Mg(OCH3)2/NH4Cl. 18
Protease, Alcalase-CLEA and Lipases from different sources are also used for regioselective transformation of such esters to amide in milder conditions.19 Due to high nucleophilicity of aluminium amides, lithium aluminium hydride-amine complexes and dimethyl aluminium amides are proved to be useful reagents for alkyl ester aminolysis.20 Aminolysis of ethyl cyano acetate with lithium amide21 and allyl esters with bis-lithium aryl amide is also reported later.22 Varma and Naicker have shown preparation of amides from inactive esters using potassium tertiary butoxide23 as the catalyst under microwave irradiation under solvent free condition.
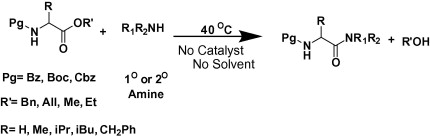
Syntheses of inactive esters are easier and cost effective than activated aromatic esters. Therefore, methods for amidation from inactive esters are always desired. Almost all the examples cited in the above mentioned publications deal with nonpeptidic substrates and harsh reaction conditions such as, highly basic or acidic media, high temperature and/or long reaction time. To the best of our knowledge, there are only two reports of catalyst and solvent free amidation procedures available at ambient temperature. However, in one case purely nonpeptidic substrates (pyridone analogs) were explored24 while in another amidation was achieved from the ester situated at the side chain carboxylic acid group of a homopolymer of aspartic acid.25 Herein we report preparation of amides from alkyl esters of N-protected amino acids at 40 °C temperature without the use of any catalyst under a solvent free condition and explored the reactivity of different amino acids by varying N-terminus protection.
For our ongoing research, we needed benzyl amides of various amino acid derivatives. Among many protocols available in literature we chose the one reported by Perreux et al.26 as they avoided the use of –COOH activating reagent, catalyst or extra solvent. We first tried to reproduce the original work using benzyl amine and phenyl acetic acid in a domestic microwave oven at 160 °C (convection heating mode) instead of a dedicated microwave reactor (Synthwave® 402 from Prolabo was used in the original article) and found the amide was produced in good yield. However, replacing phenyl acetic acid with benzoyl glycine produced only a trace amount of amide.
The relative inertness of carboxylic acid group of amino acids towards amidation prompted us to follow mild activation strategy by forming benzyl ester. Benzyl group is commonly used for C-protection of amino acids similar to methyl and ethyl esters. When benzyl ester of N-benzoyl glycine was reacted with 10 equiv of benzyl amine for 30 min at 160 °C under a microwave heating it gave corresponding amide in good yield. Thus the use of benzyl ester is more efficient and convenient compared to the methods using unprotected amino acids. This result inspired us to explore the possibility of preparation of amides from inactive esters of amino acids. At first, various parameters were optimized to find out the best condition for such reactions. There was no decrement in the yield of the product with decrease in the temperature of the microwave oven (reactions were performed at 160, 120, 100 and 40 °C). Later we repeated the reaction in a shaking incubator at 40 °C ( Scheme 1). Since incubator provides uniform heating we considered it as a better option. If desired, an oil bath or a microwave oven also can be used. Subsequently, from several trials we found that the use of 3 equiv of amine was found to be equally effective in giving corresponding amide.
After completion of the reaction (monitored by TLC), the reaction mixture was directly loaded on to a silica gel column. Our desired amide along with benzyl alcohol, which was produced during the course of the reaction and unreacted amine, was separated and collected back easily.