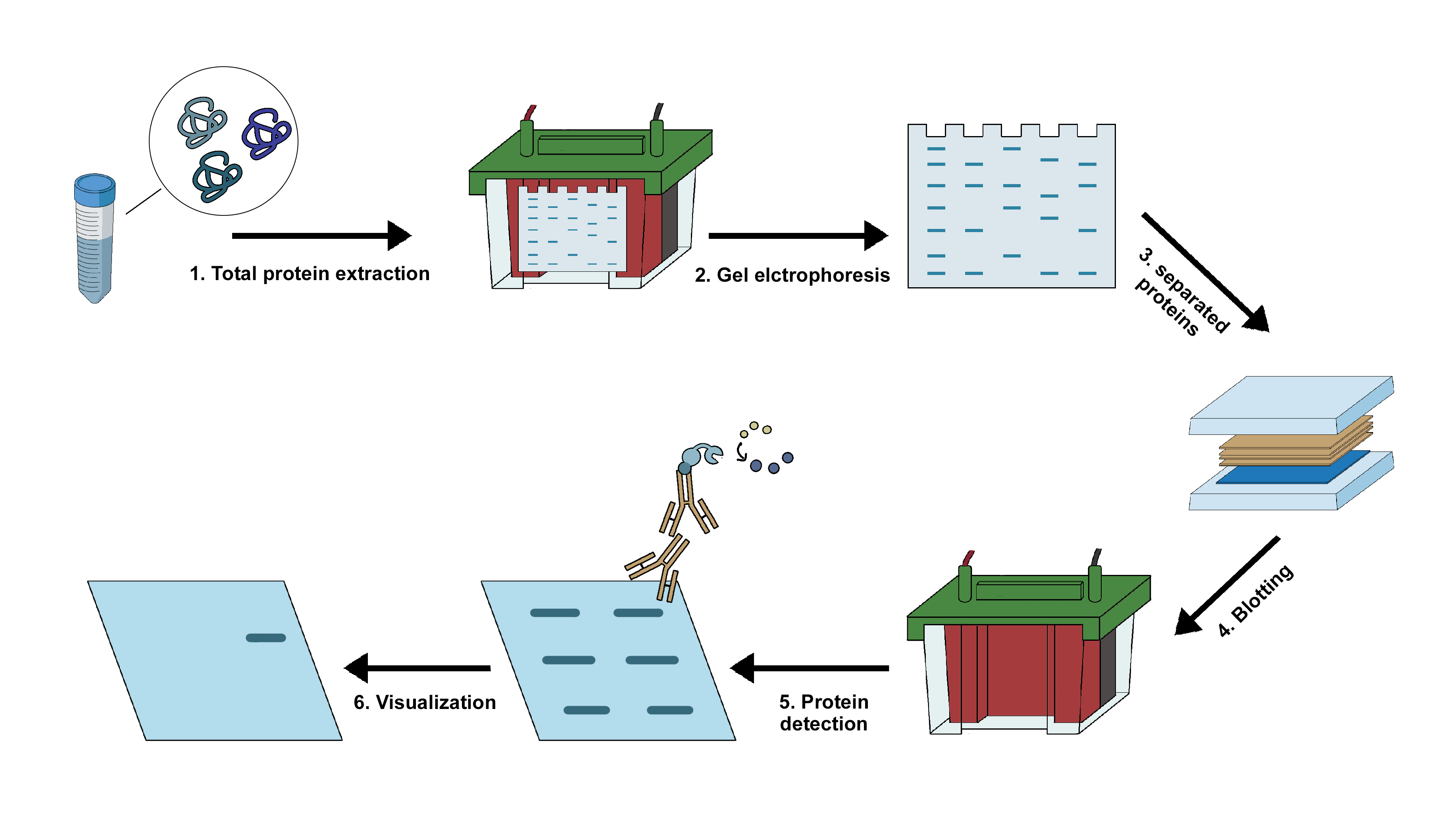
Implementation of Western Blot Analysis of the Cell Lysate using Antibody
Theory :
Western blotting is an important technique in cellular and molecular biology. This technique helps in identification of specific protein of interest from a complex mixture of proteins isolated from the cells. The three crucial steps involved in this technique includes:
- separation of proteins on the basis of their molecular weight,
- transfer of the proteins to a solid membranous support, and
- marking the protein with specific primary and secondary antibody for visualization.
The most common samples for western blot are usually cell lysates. Protein extraction is done in cold temperature in presence of protease inhibitors to prevent protein denaturation. The concentration of the extracted protein should be kept constant to ensure the sample comparison is done on equivalent basis. The sample is diluted into the loading buffer, containing glycerol that allows the sample to sink easily inside the wells of the gel followed by heating. The heating helps in breaking the higher order structures, so that the negative charge on the amino acids are not neutralized which helps in the movement of the protein in the electric field. A tracking dye usually bromophenol blue is present in the loading buffer to track the progress of separation.
The gel electrophoresis step involves two different gel types
- Stacking gel (PH6.8) has lower concentration of acrylamide making a porous gel. This allows the protein to form sharply defined bands though the separation is poor in this gel.
- Resolving gel (PH 8.8) has higher concentration of acrylamide making the pores narrower allowing the separation of proteins more precisely according to their size.
After separation, the protein mixture is transferred to a membrane using an electric field that is oriented perpendicular to the surface of the gel. The membrane is placed between the positive electrode and the gel surface, so that the negatively charged protein migrates from the gel to the membrane. This transfer is referred to as electrophoretic transfer and can be conducted under semi-dry or wet conditions. Two types of membrane are used in this process: nitrocellulose and PVDF. Nitrocellulose membrane has higher affinity for protein and has efficient retention abilities. However, this membrane cannot be used for reprobing as it is brittle in nature. PVDF membrane on the other hand provides better mechanical support and hence can both be reprobed and stored. Since the background is higher in PVDF membrane, washing should be done carefully when such membrane is concerned.
Following the transfer, the membrane is usually blocked using 5% BSA or skimmed milk diluted in TBST. Blocking is an important step in western blotting, as it prevents the nonspecific binding of the antibodies to the membrane. The concentration of the antibody depends on the manufacturer’s instruction. Putting the antibody in BSA solution allows it to be reused especially the primary antibody as it is required in higher amount compared to the secondary antibody. The washing steps after incubation with the antibodies are very crucial as it removes the unbound antibodies and minimizes the background. However, excessive washing can also reduce the signal. The membrane is detected using the secondary antibody tagged with enzyme like horseradish peroxidase (HRP), that produces signal corresponding to the position of the target protein. (1)
The data produced with a western blot is considered to be semi-quantitative because it provides relative comparison of protein levels, but not an absolute measure of its quantity. There are two reasons for such semi-quantitative quantification:
- Variations in transfer and loading rates between the samples in separate lanes which are also different on separate blots, such differences are required to be standardized before a precise comparison can be made.
- The signal generated by detection is not linear across the concentration range of the samples hence should not be used to model the concentration.